RNA Therapeutics Institute RNA Therapeutics From Concept to Clinic 2024
$30,00
This Product is shared via google drive download link, So please share your correct Gmail id while placing the order .Please note that there are no CME points or certificate associated with this course Samples for Courses Can be found here : Free Samples Here!
+ Include: 3 videos + 1 pdf, size: 84.43 GB
+ Target Audience: R&D scientists, CMC/quality leaders, translational and clinical teams, regulatory/strategy professionals,
manufacturing/operations managers
+ Information:
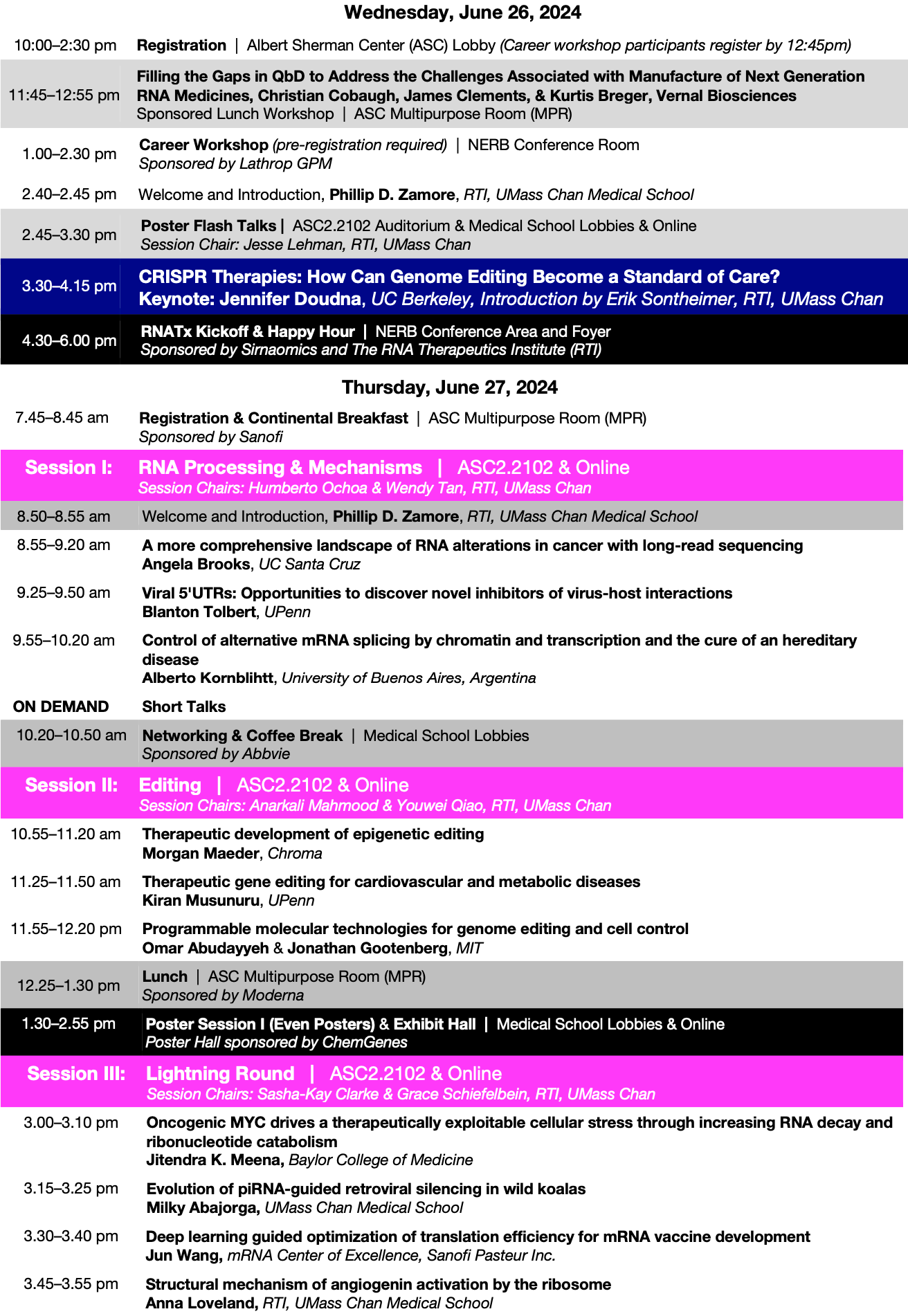
A translational, bench-to-bedside program covering the full lifecycle of RNA medicines. Faculty connect platform science (siRNA, ASO, mRNA, saRNA) with delivery, CMC, regulatory strategy, and clinical development to help teams move candidates efficiently and safely toward approval.
What You Will Learn
-
Modality selection and design: siRNA vs. ASO vs. (sa)mRNA—chemistries, modifications, and stability
-
Delivery systems: LNPs, GALNAc conjugates, polymers/peptides, local vs. systemic administration
-
Preclinical translation: in vitro/in vivo models, PK/PD, biodistribution, immunogenicity and off-target risk
-
CMC fundamentals: process development, analytics, release/stability testing, scale-up and tech transfer
-
Clinical development: FIH design, dose selection, biomarkers, patient selection, endpoints, and safety monitoring
-
Regulatory & quality: IND/IMPD packages, comparability, potency assays, pharm tox expectations, and risk management
-
Manufacturing operations: raw materials (lipids, nucleotides, enzymes), single-use workflows, contamination control
-
Data & tools: sequence optimization, secondary structure prediction, bioinformatics/AI for target and off-target assessment
-
Access & value: manufacturing cost drivers, IP strategy, partnerships, and commercialization pathways
Event Details
-
Format: Case-based lectures, panel Q&A, and short technical workshops
-
Structure: Concept/design → delivery/PKPD → CMC/manufacturing → clinical/regulatory → commercialization
-
Takeaways: Checklists, study-design templates, and CMC/quality worksheets for immediate use
Who Should Attend
R&D scientists, CMC/quality leaders, translational and clinical teams, regulatory/strategy professionals, manufacturing/operations managers, platform and biotech founders, investors, and trainees entering the RNA space.
Why Attend
-
Replace trial-and-error with validated design and development playbooks
-
Anticipate regulatory and manufacturing pitfalls early to de-risk timelines
-
Bring back practical tools to align discovery, CMC, and clinical teams around the same plan
+ Topics:
* Detail:

Related products
ONCOLOGY-HEMATOLOGY
USCAP Hematopathology Tasting Menu: A Sampling of Delightful Diagnostic Challenges 2021 (CME VIDEOS)
PULMONARY /RESPIRATORY
ONCOLOGY-HEMATOLOGY
2022 ASTRO Annual Refresher Course On Demand (Interactive way)
ENDOCRINE / NUTRITION
ONCOLOGY-HEMATOLOGY
2023 Hematology and Oncology Best Practices On demand full course (8 Days)